Chapter No.1: Chemical Equilibrium -Chemistry For Class X (2022 and Onward) - Text Book Exercises ( Section "B" & "C" and Test Yourself)
Chapter No.1: Chemical Equilibrium -Chemistry For Class X (2022 and Onward) - Text Book Exercises ( Section "B" & "C" and Test Yourself)
Go To Index
Chemical Equilibrium
Text Book Exercises
SECTION- B: SHORT QUESTIONS
1. Define chemical equilibrium with example.Ans: Chemical Equilibrium:
The reversible chemical reaction never goes to completion because product reacts and reproduce reactants again and take place in forward and reverse direction. This state in which forward reaction rate and reverse reaction rate are equal known as equilibrium.
Equilibrium means a 'balance'. Equilibrium exists in many ways in our surroundings.
"It is a state where the rate of the forward reaction is equal to the rate of backward reaction."
OR
It is also defined as "A state where the concentration of reactant and product remains constant."Example:
H2(g) + I2(g) ⇌ 2HI(g)
2. Why chemical equilibrium is dynamic?
Ans: Chemical Equilibrium Is Dynamic:
We know that chemical equilibrium can be written by general equation as:
aA + bB ⇌ cC + dD
The reaction rate depends on the concentration of the reactants. At the beginning the quantity of reactant is higher, and the rate of forward reaction is higher.Forward rate:
Rf = Kf [A]a [B]b
As the product is formed, the reverse reaction is then able to occur.Backward rate:
Rr = Kr [C]c [D]d
As the reactant amount decreases, the rate of reactant transformation also decreases, and the rate of product formation decreases. After a certain time, the concentrations of reactants and products become constant, and equilibrium is established.Rate of forward reaction = Rate of reverse reaction
Rf = Rr
Kf [A]a [B]b = Kr[C]c [D]d
Upon arrangement,Rf = Rr
Kf [A]a [B]b = Kr[C]c [D]d
Where Kc is called equilibrium constant for the reaction.
The rate of both forward and reverse reaction becomes equal upon reaching the equilibrium point. So, there is no observable changes although both forward and reverse reactions occurring. The reaction has not stopped. Hence, it has proved that chemical equilibrium is dynamic in nature.
3. When writing an equation, how is a reversible reaction distinguished from irreversible reaction?
Ans: In writing an equation irreversible reaction is written with single right headed arrow (→). While reversible reaction means the products also react with each other and become reactant once again, so we write it with double half headed arrows (⇌) with opposite directions. The right arrow is represnting the reaction from reactant to product and the left arrow represents the reaction from products to reactant.
OR
In other words,The direction of a reaction can be predicted by type of arrow; single headed arrow (→) used for irreversible reactions and a double half headed arrows (⇌)are used for reversible reaction, that never goes to completion.
4. Write an equilibrium equation of monatomic carbon and a molecule of oxygen as reactant and carbon monoxide as product.
Ans: EQUATION
2Cs + O2(g) ⇌ 2CO(g)
5. Outline the characteristics of reversible reaction.
Ans: CHARACTERISTICS OF REVERSIBLE REACTION:
- It is always directed from right to left in a chemical reaction.
- Product produce reactant (Reactants ← Products).
- Initially rate is slow but gradually speed up.
- Reversible reactions always attain equilibrium and never proceed to completion under certain condition of temperature and pressure.
- Dynamic equilibrium is established.
6. Distinguished between reversible and irreversible reaction.
Ans: Difference Between Reversible And Irreversible Reaction
| S.No. | Reversible Reaction | Irreversible Reaction |
|---|---|---|
| 1. | It can reverse in suitable condition. | It can reverse in suitable condition. |
| 2. | Both forward and backward reactions take place simultaneously. | It is unidirectional, it proceeds only in a forward direction. |
| 3. | It attains equilibrium. | equilibrium is not attained. |
| 4. | The reactant can not be converted completely into products. | The reactant can be converted completely into products. |
| 5. | It is relatively slow. | It is fast |
| 6. | Double half headed arrows (⇌)are used for reversible reaction | Single headed arrow (→) used for irreversible reactions |
7. State law of mass action. How is the active mass is represented?
Ans: LAW OF MASS ACTION:
Statement:
The law of mass action state that:
"The rate of a reaction is directly proportional to the product of the concentration of each reactant."
REPRESENTATION OF ACTIVE MASS:
The concentration of reacting substance is called Active mass. The unit of active mass is mol and its value is expressed in square brackets [ ].
Example: [O2] = 0.127 mol.dm-3
8. Why equilibrium constant may or may not have unit? Justify with example.
Ans: Unit Of Equilibrium Constant:
At Equilibrium Position Equilibrium Constant Has No Unit:
Rate of the forward reaction = Rate of the backward reaction
An equal number of moles on both sides of the equation has no unit in Kc, because Kc expression uses concentration units that cancel. The unit of concentration is mol.dm-3.
Consider a reaction:
CO2(g) + H2(g) ⇌ CO(g) + H2O(g)
If Reactant And Products Concentration Are Not Equal Kc has unit:
For reactions when the number of moles of reactants and product are not equal, Kc has a unit. Let us consider the following reaction:
N2(g) + 3H2(g) ⇌ 2NH3(g)
9. How direction of a reaction can be predicted if Kc is known to you.
Ans: Direction Of A Chemical Reaction:
When dealing with reversible reactions, it is critical to determine the reaction's direction at any given time. The reaction quotient, Qc, can help make such predictions.
Under non-equilibrium condition, reaction quotient 'Qc' is define as the ratio of the product of active masses of reactant and products raised to the respective stoichiometric coefficients in the balanced chemical equation to that of the reactant.
It has the some mathematical structure as Kc. Comparing Kc and Qc values predicts reactions direction. We have three categories:
- If Qc = Kc:
The actual product and reactant concentrations are equal to the equilibrium concentrations, and the system is stable. - If Qc < Kc:
Then there is increase in product concentration for equilibrium. So the forward reaction occurs, forming additional products. - If Qc > Kc:
There is decrease in product concentration & to achieve equilibrium. As, the process reverses, forming more reactants.
10. Write equilibrium constant expression for the following equations:
i) N2 + 2O2 ⇌ 2NO2
ii) N2 + H2 ⇌ 2NH3
iii) H2 + Br2 ⇌ 2HBr
Ans: i) N2 + 2O2 ⇌ 2NO2
ii) N2 + H2 ⇌ 2NH3
iii) H2 + Br2 ⇌ 2HBr
SECTION-C: DETAILED QUESTIONS
1. Describe dynamic equilibrium with two examples.Ans: Dynamic Equilibrium:
The reaction rate depends on the concentration of the reactants. At the beginning the quantity of reactant is higher, and the rate of product formation is the higher. As the reactant amount decreases, the rate of reactant transformation also decreases, and the rate of product formation decreases. After o certain time, the concentrations of reactants and products become constant, and this state is called dynamic equilibrium.
Rate of forward reaction = Rate of reverse reaction
In a reversible reaction, dynamic equilibrium is established before the completion of reaction. The rate of both forward and reverse reaction becomes equal upon reaching the equilibrium point.
Graph:
The following graph which is of concentrations vs. time, shows that the concentrations of both reactants and product becomes constant at equilibrium.
Example No.1: Formation of Hydrogen Iodide
An example of a reaction at equilibrium is a reaction of hydrogen and iodine in a closed container to produce hydrogen iodide. At the start of the reaction, there is a high concentration of hydrogen and iodine and, after that, the concentration decreases as hydrogen iodide is formed. The concentration of hydrogen iodide increases as the forward reaction proceeds. As hydrogen iodide is formed, the reverse reaction is then able to occur.
H2(g) + I2(g) ⇌ 2HI(g)
So, there is no observable changes although both forward and reverse reactions occurring. The reaction has not stopped but reached dynamic equilibrium.
Example No.2: Formation of Ammonia
Let us take an example of manufacturing of ammonia.When one mole of nitrogen gas reacts with three moles of hydrogen it produces two moles of ammonia gas. This is known as 'Forward reaction'.
N2(g) + 3H2(g) → 2NH3(g)
In contrast, two moles of ammonia gas may also be converted into one mole of nitrogen and three moles of hydrogen. This is known as 'Reverse reaction'.N2(g) + 3H2(g) ← 2NH3(g)
When both of these reactions are written together as a reversible reaction, they are represented as:N2(g) + 3H2(g) ⇌ 2NH3(g)
So, there is no observable changes although both forward and reverse reactions occurring. The reaction has not stopped and reached dynamic equilibrium.2. State law of mass action. Derive an expression for equilibrium constant.
Ans: LAW OF MASS ACTION:
Statement:
The law of mass action state that:
"The rate of a reaction is directly proportional to the product of the concentration of each reactant."
Explanation:
The rate at which a substance reacts is directly proportional to its active mass and the rate of a reaction is directly proportional to the product of the active masses of the reacting substances. The law of mass action also suggests that the ratio of the reactant concentration and the product concentration is constant at a state of chemical equilibrium.
Derivation Of Equation For Equilibrium Constant:
Let us apply law of mass action on a hypothetical reversible reaction.
aA + bB ⇄ cC + dD
Forward Reaction:First let us discuss forward reaction, where A and B are reactants whereas 'a' and 'b' are number of moles needed to balance a chemical equation. The rate of forward reaction according to law of mass action is:
Rf ∝ [A]a [B]b
Rf = Kf [A]a [B]b
Where Kf is the rate constant for forward reaction.Reverse reaction:
Likewise, rate of reverse reaction is directly proportional to product of molar concentrations of C and D whereas 'c' and 'd' are number of moles needed to balance a chemical reaction.
Rr ∝ [C]c [D]d
Rr = Kr [C]c [D]d
Where Kr is the rate constant for reverse reaction.At Equilibrium:
We know that, at equilibrium rate of forward and reverse reaction becomes equal. So,
Rf = Rr
Putting the values of Rf and Rr, we have:Kf [A]a [B]b = Kr [C]c [D]d
By taking constants on L.H.S and variables on R.H.S, we haveWhere Kc is called equilibrium constant.
Hence proven that law of mass action describe relation between active masses of reactants and products with rate of reaction. All the reversible reactions can be expressed in this form.
3. Describe the characteristics of equilibrium constant.
Ans: Characteristics Of Equilibrium Constant Expression
- Important characteristics of equilibrium constant expression are as follows:
- Kc only works in equilibrium.
- It represents the equilibrium concentration of the reactant and product in mol.dm-3
- Kc is independent of reactant and product concentrations.
- Kc varies with temperature.
- Kc is a balanced chemical equation coefficient. In a balanced chemical equation, each reactant and product has a concentration equal to its coefficient.
- Kc represents equilibrium position. If Kc is larger than 1, the reaction is forward. If Kc is less than 1, the reaction is a reverse reaction.
- Equilibrium constant Kc is a ratio of reactant to product that is utilized to define chemical behavior.
4. How can you predict the following stages of a reaction by comparing the values of Kc and Qc.
- Net reaction proceeds in forward direction.
- Net reaction proceeds in reverse direction
If Qc < Kc:
Then there is increase in product concentration for equilibrium. So the forward reaction occurs, forming additional products.
Example:
In the beginning of reaction, when one mole of nitrogen gas reacts with three moles of hydrogen it produces two moles of ammonia gas. 'Forward reaction' proceed.
N2(g) + 3H2(g) → 2NH3(g)
ii) Net reaction proceeds in reverse direction
If Qc > Kc:
There is decrease in product concentration & to achieve equilibrium. As, the process reverses, forming more reactants.
Example:
After formation of ammonia, two moles of ammonia gas may also be converted into one mole of nitrogen and three moles of hydrogen. 'Reverse reaction' proceed.
N2(g) + 3H2(g) ← 2NH3(g)
5. Predict which system at equilibrium will contain maximum amount of product and which system will contain maximum amount of reactant?
- 2CO2(g) ⇌ 2CO(g) + O2(g) Kc(927 C) = 3.1x10-18 mol.dm-3
- 2O3(g) ⇌ 3O2(g) Kc(298K) = 5.9 x 1055 mol.dm-3
- If Kc is very large, ~1000 or more, we will have mostly product species present at equilibrium.
- If Kc is very small, ~0.001 or less, we will have mostly reactant species present at equilibrium.
- If Kc is between 0.001 and 1000, we will have a significant concentration of both reactant and product species present at equilibrium.
strongly favour the forward direction to make products → very large Kc, strongly favour the backward direction to make reactants → very small Kc, or somewhere in between.
Therefore, system (b) will contain the maximum amount of product.
TEST YOURSELF
1. Write down forward and reverse reactions for the following.N2(g) + O2(g) ⇌ 2NO
2SO2(g) + O2(g) ⇌ 2SO3(g)
COCl2(g) ⇌ CO(g) + Cl2(g)
Ans: (i)
Forward Reaction: N2(g) + O2(g) ⟶ 2NO
Reverse Reaction: N2(g) + O2(g) ← 2NO
(ii)
Forward Reaction: 2SO2(g) + O2(g) ⟶ 2SO3(g)
Reverse Reaction: 2SO2(g) + O2(g) ← 2SO3(g)
(iii)
Forward Reaction: COCl2(g) ⟶ CO(g) + Cl2(g)
Reverse Reaction: COCl2(g) ← CO(g) + Cl2(g)
2. Define the term active mass?
Ans: ACTIVE MASS:
The concentration of reacting substance is called Active mass.
Unit:
The unit of active mass is mol.dm-3 or mol/L and its value is expressed in square brackets.
3. Figure out coefficients for given hypothetical reaction.
9X(g) + Y3(g)`⇌ 3X3Y(g)
Ans: Coefficients Of X = 9Coefficients Of Y3 = 1
Coefficients Of X3Y = 3
4. Write down Kc equation for given reaction:
- S(s) + O2(g) ⇌ SO2(g)
- SO2(g) + NO2(g) ⇌ NO(g) + SO3(g)
- NH4Cls ⇌ NH3(g) + HCl(l)
(i) S(s) + O2(g) ⇌ SO2(g)
(ii) SO2(g) + NO2(g) ⇌ NO(g) + SO3(g)
(iii) NH4Cls ⇌ NH3(g) + HCl(l)
5. The value of Kc for the following reaction at 717K is 48.
H2(g) + I2(g) ⇌ 2HI(g)
At a particular instant, the concentration of H2, I2 and HI are found to be 0.2 molL-1, 0.2 molL-1, and 0.6 molL-1 respectively. Calculate reaction quotient for given reaction. Also predict direction of reaction.Ans: Solution:
Given:
- Kc = 48 at 717K
- [H2] = 0.2 molL-1
- [I2] = 0.2 molL-10.2 molL-1
- [HI] = 0.6 molL-1
At a given time the reaction quotient Qc for given reaction will be given by the expression:
Therefore Qc < Kc
Ans: Qc < Kc, so reaction will proceed in forward direction.
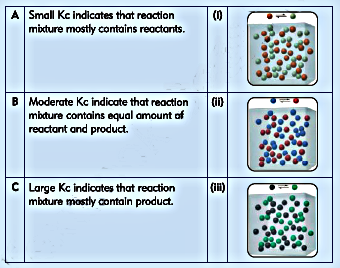
6. Match each of the following statement with appropriate diagram.
Ans: A- (ii) , B- (i), C- (iii)
Do You Know
Q.1: How equilibrium occurs in our Body?Ans: Equilibrium in our Body:
Equilibrium is present in our bodies. Hemoglobin is macromolecule that transports oxygen around our bodies. Without it we would not survive. The haemoglobin has to be able to take up oxygen, but also to release it and this Is done through changes in the chemical equilibrium of this reaction in different places in our bodies.
Q.2: In which groups equilibrium constant exist?
Ans: Equilibrium constants exist for certain groups of equilibria, such as for weak acids, weak bases, the autoionization of water, and slightly soluble salts.
Q.3: Write down few lines about society, technology and science.
Ans: Society, Technology and Science:
The atmosphere is composed of nitrogen, oxygen, carbon dioxide, methane, nitrous oxide and ozone but the nitrogen and oxygen gases are the most important port of the atmosphere. They are 99% of the atmosphere and use to manufacture chemicals such as nitrogen is used for preparation of ammonia and ammonia is used to prepare nitrogenous fertilizers. Oxygen is used for preparation of sulphur dioxide and sulphur dioxide is used to prepare sulphuric acid.










.png)
.png)
.png)


Comments
Post a Comment
If you have any doubts, Please let me know .